Table of Contents
Pregnancy during Coronavirus Outbreak:
Reasons for considering Mesenchymal Stem Cell Therapy for COVID-19
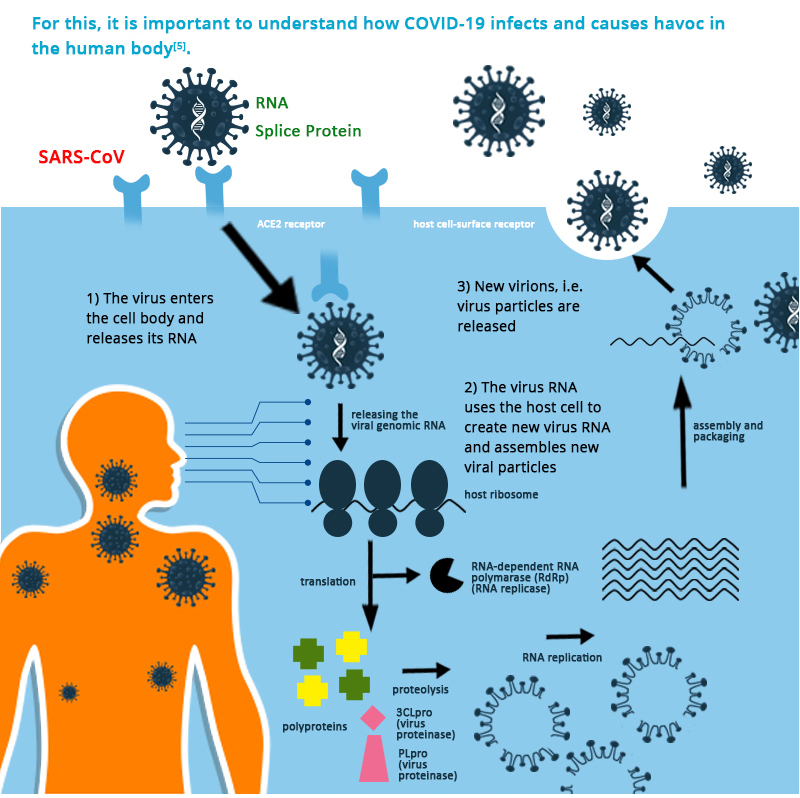
Basically, the S protein, on COVID-19, recognises the Angiotensin Converting Enzyme 2 (ACE2) receptor, which is naturally present on the surface or membrane of normal cells of lungs, heart, liver, digestive organs, kidneys, almost all endothelial cells and smooth muscle cells. Upon attachment of the COVID-19 with the ACE2, both fuses to constitute a conduit, through which the viral RNA (virus’s genetic material) is injected into the human cell. This invasion hijacks the human cells protein and forces them to produce different parts of the COVID-19. These cells get distended with fully assembled viruses, to such an extent, that they burst – not only killing the host cell in the process but also releasing all the viruses into the body, free to infect new cells. As the cells die, they also release cytokines, which warn the cells in the periphery. Due to these cytokines, the cells in the vicinity die, setting up a barrier (barren shells) between live normal cells and the virus-infected cells, in a natural attempt to lock down or localise the infection. If many cells are affected at the same time, the huge amount of bad cytokines, cook up a catastrophic situation called “Cytokine Storm”, which tends to kill plenty of cells intrinsically, finally killing the human. This is the second cause of death in COVID-19 infection in addition the individual organ failures. This is how the cycle of infiltration, hijacking, sinking of the hijacked and further rampage continues.
The Italian College of Anesthesia, Analgesia, Resuscitation and Intensive Care hase reported guidelines to treat COVID-19 patients with stem cells in the hope of decreasing the number of patients going to the ICU, and also relatively quickly getting them out of the ICU[6].
Since there are no FDA-approved vaccines or drugs to treat COVID-19, FDA has approved clinical trials for vaccines and drugs but it would take several months before they are available in the market. In such a scenario, where clinical trials using MSCs have been approved by FDA, and also promising results seen from other studies using MSCs from the umbilical cord (“UC-MSCs”) in helping the recovery of critically-ill patients at multiple centres, could probably be here to SAVE THE DAY[1,2,6-14].
Subsequent to the results reported from clinical trials conducted in China, preliminary results of stem cell experiments to treat the COVID-19 conducted across the country suggested that the technology was “safe and effective”.
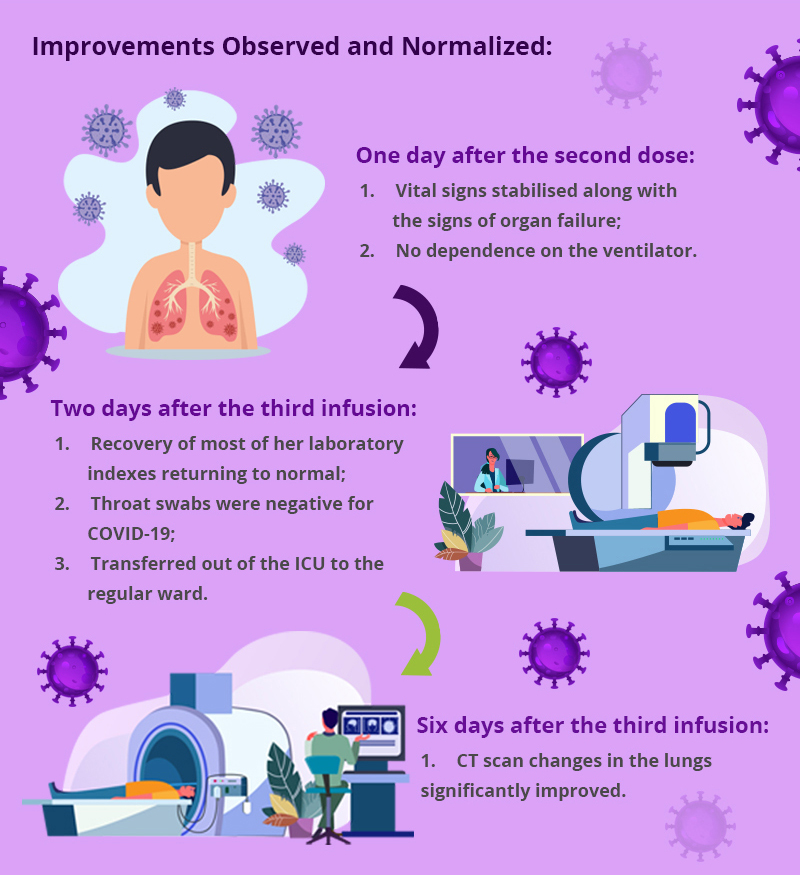
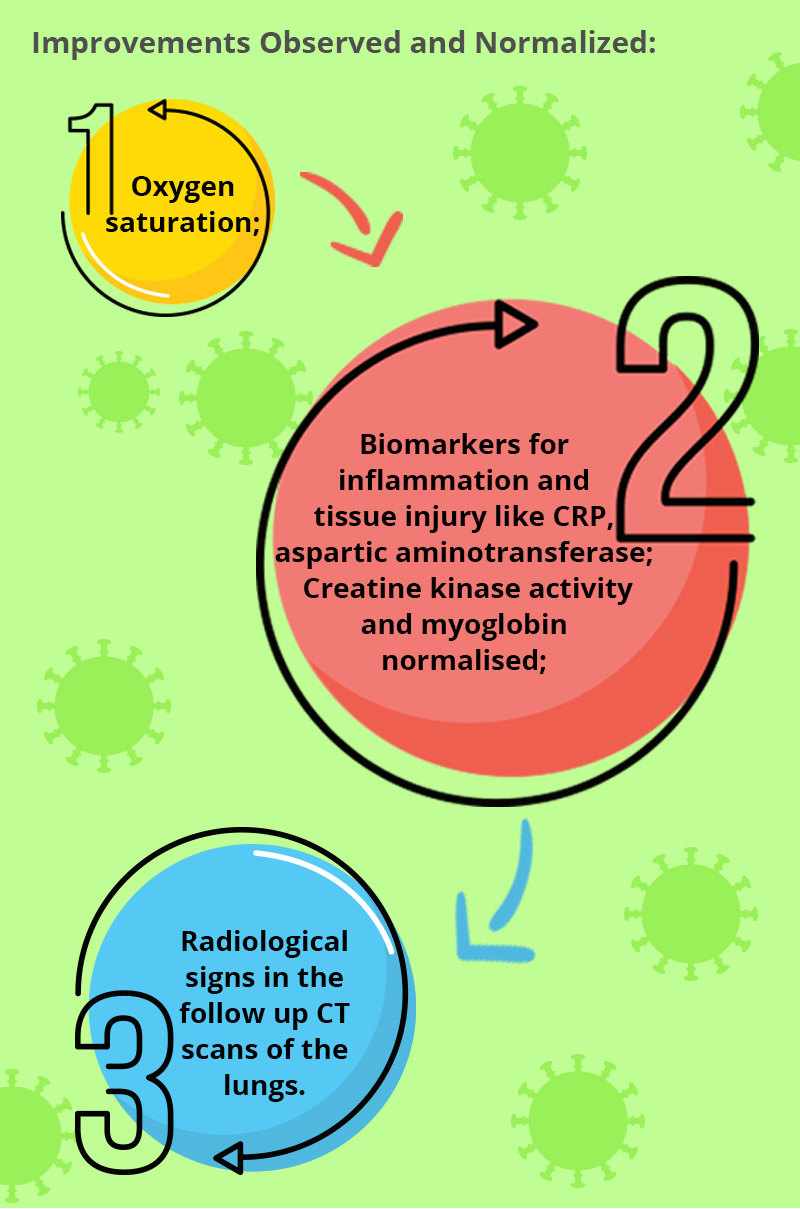
I. The first COVID-19 case treated with UC-MSCs was reported from China. A 65-year-old with severe pneumonia, respiratory failure and multiorgan failure requiring mechanical ventilation. She was treated with 3 doses of allogeneic UC-MSCs, three days apart. Each dose contained 50 million allogeneic UC-MSCs. Surprisingly, for a patient who was not responding to conventional therapy, improvements were observed in several parameters.
II. Similar results were observed in another recently published small-scale clinical trial, where 7 patients (1 critically serious, 4 serious and 2 common) infected with the COVID-19 were given one dose of stem cell therapy each. These patients were compared with 3 other patients in the control group (3 serious) who did not receive any stem cell dose. All these patients were not responding to standard treatment. However, on follow-up for 14 days, all 7 patients in the treatment group who were administered with stem cell dose, recovered and did not exhibit any complications.
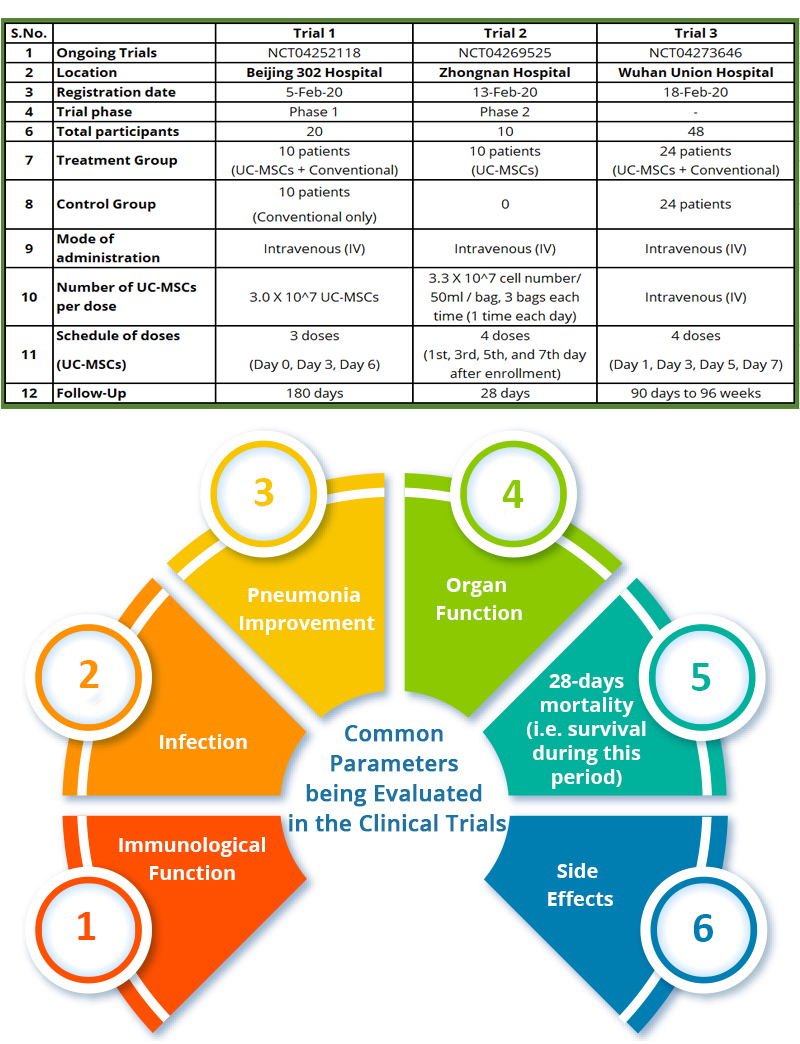
Registered and Ongoing Clinical Trials using UC-MSCs for COVID-19
Why UC-MSCs could be a preferred choice?[6,15-22]
MSCs have been chosen as a treatment option, due to its mode of action in treating infections.
- MSC therapy can theoretically inhibit the overactivation of the immune system and promote endogenous repair by improving the microenvironment.
- After entering the human body through intravenous infusion, part of the MSCs accumulate in the lung, which could potentially improve the pulmonary microenvironment, protect alveolar epithelial cells, prevent pulmonary fibrosis and improve lung function.
- MSCs play a positive role mainly in two ways, namely immunomodulatory effects and differentiation abilities.
- MSCs exert their antimicrobial effects through indirect and direct mechanisms. Indirectly, they influence the role of host immune response against pathogens, by increasing the activity of phagocytes; and directly, by the secretion of antimicrobial peptides and proteins (AMPs), and by the expression of molecules such as indoleamine 2,3-dioxygenase (IDO) and interleukin (IL)-17.
- MSCs have been found to constitutively express four AMPs: cathelicidin LL-37, human β-defensin-2 (hBD-2), hepcidin, and lipocalin-2 (Lcn2), which can be further modulated during infection and inflammation.
AMPs-mediated cell killing occurs by disrupting membrane integrity, by inhibiting protein, DNA or RNA synthesis, and by interacting with certain intracellular targets.
References:
- Ruth Williams, Are mesenchymal stem cells a promising treatment for COVID-19?, The Scientist website, the-scientist.com/news-opinion/are-mesenchymal-stem-cells-a-promising-treatment-for-covid-19–67402 , 9 April 2020; Accessed 14 April 2020.
- Hope Biosciences receives FDA approval to commence first stem cell clinical trial for protection against COVID-19, BioSpace website, biospace.com/article/releases/hope-biosciences-receives-fda-approval-to-commence-first-stem-cell-clinical-trial-for-protection-against-covid-19/, 6 April 2020, Accessed 14 April 2020.
- Coronavirus (COVID-19) Update: Daily Roundup March 20,2020, FDA website, fda.gov/news-events/press-announcements/coronavirus-covid-19-update-daily-roundup-march-30-2020, 30 March 2020; Accessed 14 April 2020.
- COVID-19 Coronavirus Pandemic Case Count, Worldometer website. worldometers.info/coronavirus/; Accessed 14 April 2020.
- Koester V, Coronavirus entering and replicating in a host cell, Chemistry Views website, chemistryviews.org/details/ezine/11225161/Coronavirus_Entering_and_Replicating_in_a_Host_Cell.html, 3 March 2020, Accessed 14 April 2020.
- Atluri S, Manchikanti L, Hirsch J. Expanded umbilical cord mesenchymal stem cells (UC-MSCs) as a therapeutic strategy in managing critically ill COVID-19 patients: The case for compassionate use. PAIN PHYSICIAN. 2020;23():E71-E83.
- Liang B, Chen J, Li T, et al. Clinical remission of a critically ill COVID-19 patient treated by human umbilical cord mesenchymal stem cells. ChinaXiv 2020;202002.00084.
- gov website. Mesenchymal stem cell treatment for pneumonia patients infected with 2019 novel coronavirus. ClinicalTrials.gov Identifier: NCT04252118. https://clinicaltrials.gov/ct2/show/NCT04252118, Accessed 15 March 2020.
- gov website. Umbilical cord (UC)-derived mesenchymal stem cells (MSCs) treatment for the 2019-novel coronavirus (nCOV) pneumonia. ClinicalTrials.gov Identifier: NCT04269525. https://clinicaltrials.gov/ct2/show/NCT04269525?term=stem+cells&cond=Corona+Virus+Infection&draw=2; Accessed 15 March 2020.
- gov website. Study of human umbilical cord mesenchymal stem cells in the treatment of novel coronavirus severe pneumonia. ClinicalTrials.gov Identifier: NCT04273646. https://clinicaltrials.gov/ct2/show/NCT04273646?term=stem+cells&cond=Corona+Virus+Infection&draw=2; Accessed 15 March 2020.
- gov website. Therapy for pneumonia patients infected by 2019 novel coronavirus. ClinicalTrials.gov Identifier: NCT04293692. https://clinicaltrials.gov/ct2/show/NCT04293692?term=stem+cells&cond=Corona+Virus+Infection&draw=2&rank=7; Accessed 15 March 2020.
- Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 395:497-506.
- Leng Z, Zhu R, Hou W. Transplantation of ACE2 mesenchymal stem cells improves the outcomes of patients with COVID-19 pneumonia. Aging Dis.2020;11:216-228.
- Behnke J, Kremer S, Shahzad T, et al. MSC-based therapies-new perspectives for the injured lung. J Clin Med. 2020;3:9.
- Cruz FF, Rocco PRM. The potential of mesenchymal stem cell therapy for chronic lung disease. Expert Rev RespirMed. 2020;14:31-39.
- Li D, Liu Q, Qi L, Dai X, Liu H, Wang Y. Low levels of TGF-β1 enhance human umbilical cord-derived mesenchymal stem cell fibronectin production and extend survival time in a rat model of lipopolysaccharide-induced acute lung injury. Mol Med Rep. 2016;14:1681-1692.
- Iyer SS, Co C, Rojas M. Mesenchymal stem cells and inflammatory lung diseases. Panminerva Med. 2009;51:5-16.
- Alcayaga-Miranda F, Cuenca J, Khoury M. Antimicrobial activity of mesenchymal stem cells: Current status and new perspectives of antimicrobial peptide-based therapies. Front Immunol. 2017;8:339.
- Predictive Technology Group Addresses Use of Mesenchymal Stem Cells in Treatment of Secondary Issues Related to Coronavirus. GlobeNewswire website, https://finance.yahoo.com/news/ predictive-technology-group-addressesmesenchymal-154552966.html, March 17, 2020.; Accessed 14 April 2020.
- Krasnodembskaya A, Song Y, Fang X, et al. Antibacterial effect of human mesenchymal stem cells is mediated in part from secretion of the antimicrobial peptide LL-37. Stem Cells. 2010;28:2229-2238.
- Arutyunyan I, Elchaninov A, Makarov A, Fatkhudinov T. Umbilical cord as prospective source for mesenchymal stem cell-based therapy. Stem Cells Int. 2016;2016:6901286.
- Weiss ML, Troyer DL. Stem cells in the umbilical cord. Stem Cell Rev. 2006;2(2):155-62.
- Nagamura-Inoue T, He H. Umbilical cord-derived mesenchymal stem cells: Their advantages and potential clinical utility. World J Stem Cells. 2014 Apr 26;6(2):195–202.