Main navigation
Additional Services Offered
Colony-Forming Unit (CFU) Assay
What is CFU assay?
When a patient needs treatment using cord blood stem cells, their bone marrow is often destroyed by chemotherapy or radiotherapy (myeloablation). This process wipes out the cells that create red blood cells, white blood cells, and platelets. Cord blood stem cells are then used to rebuild these cells.
But how can we be sure the stem cells will work properly? To check this, every cord blood unit is tested to see if it can repopulate the bone marrow with active cells. This test is called the CFU assay.
The CFU assay shows if a single stem cell can turn into different types of blood cells and how many of these cells it can produce. Simply knowing that cells are alive (viability) isn’t enough; we need to know if they can function properly, which the CFU Assay confirms.
Therefore, it's important not just to store cord blood but also to check its capacity to grow and function both before storage and after retrieval. This ensures the treatment will work effectively.
Why is CFU Assay recommended?
Cord blood units have been chosen for transplants based on basic criteria like cell count and viability. However, even the best units sometimes fail, with 20–24% not working as expected. Viability only shows that cells are alive, not if they are functional.
This led to the need for another test, the CFU Assay, to ensure stem cells will perform their function properly. The CFU Assay is now recommended as part of the standard testing process for cord blood units.
Why should you opt for CFU Assay at the time of storage?
Research from Duke University showed that the quality and potency of stem cells can change during freezing and thawing. The Cord Blood APGAR measures these changes, predicting how well the stem cells will work after being thawed. This research suggests that potency testing with the CFU assay should be done both before freezing and at the time of transplant to ensure quality from the start.
How is CFU assay done?
A set number of stem cells from umbilical cord blood are placed in a special growth medium. Under controlled conditions, these cells grow into all the different types of blood cells over about 14 days. A detailed report, including images of the grown cells, is then provided. This test shows how well the stem cells are expected to work in the body.
This test is an ex vivo representation of the expected outcome - engraftability, of the haematopoietic stem cells in vivo.
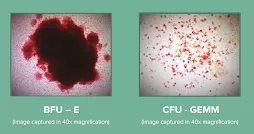
BFU-E: Burst forming unit-erythroid
CFU-GEMM: Colony-Forming Unit-Granulocyte, Erythrocyte, Monocyte/macrophage, Megakaryocyte
Reference:
1) Ivan N. Rich. Improving Quality and Potency Testing for Umbilical Cord Blood: A New Perspective. Stem Cells Translational Medicine 2015;4:967–973
2) Page et al. Total Colony-Forming Units Are a Strong, Independent Predictor of Neutrophil and Platelet Engraftment after Unrelated Umbilical Cord Blood Transplantation: A Single-Center Analysis of 435 Cord Blood Transplants. Biol Blood Marrow Transplant 17: 1362-1374 (2011)
3) Page et al. The Cord Blood Apgar: a novel scoring system to optimize selection of banked cord blood grafts for transplantation. Transfusion. 2012 February ; 52(2): 272–283
Additional Storage Programme
As healthcare improves in India, people are expected to live longer, up to 75 years or more. The Additional Storage Programme allows parents to store umbilical cord blood stem cells for an extra 54 years, beyond the initial 21 years. This extended period starts after the child’s 21st birthday, providing longer-term secured storage.
HLA typing (at the time of storage)
About HLA
Human leukocyte antigens (HLA) are proteins in most of our cells. They help the immune system recognise which cells belong to our body and which do not.
Why is HLA typing needed?
HLA typing is an essential test for matching patients with suitable cord blood stem cells for transplants.3 The best results happen when the HLA of the patient and donor are closely matched.

There are six antigens normally tested for matching. Since cord blood stem cells are immature, there is a lower risk of complications after engraftment, especially graft vs. host disease (GVHD), a potentially serious complication that arises when the immune cells, which are part of the donated stem cells, attack the patient’s body.
That is why even a four-out-of six match is just as effective at curing patients as compared to a six-out-of six or perfect match in bone marrow transplants.
Why is HLA typing recommended at the time of storage?
HLA typing and matching take time—about six to nine weeks. Doing HLA typing at storage avoids delays when the patient needs treatment. If HLA typing is already done, matching takes only two to three weeks, saving critical time. It also prevents wasting stem cells, as the test uses leftover samples instead of essential segments needed later. Quick matching can significantly improve transplant outcomes.
Reference:



